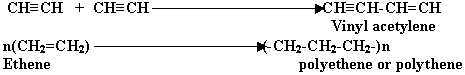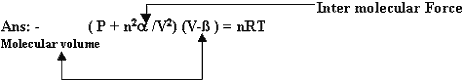| Model Paper-1
Chemistry
CBSE (2001)
Q 1.Arrange the Electron (e), Proton (p), Neutron (n)
& Alpha particles (a) with increasing order of their value of e/m
(charge/mass)? (1 mark)
Ans. - Neutron (n), Alpha (a), Proton (p), Electron (e)
Q 2. How does Cl from Cl- ion differ from each other? (1mark)
Ans. In numbers of electron.
Explanation: - Cl + e- Cl-
Cl-
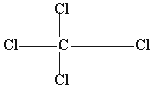
Q 3.Why is NaCl electrovalent while CCl4 a covalent compound?
(1mark)
Ans. Electrovalent bond is formed by the tr
Ansfer of electron from
one atom (metal) to another atom (non-metal). Since in NaCl bond is formed
by tr
Ansfer of electron from Na to Cl, that's why it has electrovalent
bond.
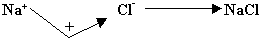
Covalent bond is formed by the sharing of electrons between two or more
atoms (non- metals). Since CCl4 is formed by the sharing of
electron between carbon & chlorine, that's why CCl4 has
a covalent bond.
Q 4.What is diagonal relationship? (1 mark)
Ans. The first element of a group often shows resemblance to the
second element of the neighbouring group on the right. This type of relationship
is called diagonal relationship. Eg. Lithium and Magnesium.
Q 5.What do you understand by the term " Polymerisation"?
(1mark)
Ans. It is a union of two or more molecules of same type to form
a new big molecule without any elimination. The molecules are called "Polymers"
and this phenomenon is called "Polymerisation".
Illustration: - NH4Cl+Cu2Cl2(as catalyst)
· 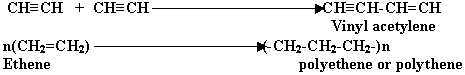
Q 6. Write Van der Waal equation? (1mark)
Inter molecular Force
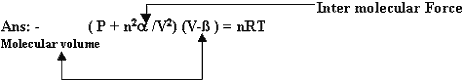
Q 7.Write the name of the atomic reactor in which graphite is used
as moderator ? (1mark)
Ans. "Atomic pile"
Q 8.Calculate the number of electrons present in 1.6 gram of CH4?
(1mark)
Ans: - 1.6 gm of CH4 = 1.6 /16 mole of CH4 = 10-1 mole of methane
= 6.023 X 1022 molecules of methane = 6.023 X 1022 X 10 electrons = 6.023
X 1023 electrons.
(Here 1 molecules of methane contain one carbon atom & 4 H-atoms consisting
of 6+4=10 electrons)
Q 9.What will be total number of orbital in 4th orbit? (1mark)
Ans. (16)
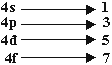
Total No. of orbitals = 16
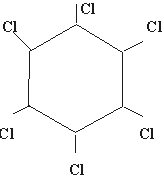
Q 10.Write the structural formula of "Gamexine"? (1mark)
Ans. The trade name of benzene hexachloride is gammexine. Its structural
formula is :
Q 11. Define: -
(i) Aufbau principle
(ii) Heisenberg uncertainty principle.(2 marks )
Ans.(i)
· Aufbau principle: -
Aufbau is a German word, which me
Ans, " to build". Hence, Aufbau
principle gives the principles building of atomic structure of elements
with electrons.
According to this principle: -
"Electrons are filled in atomic orbital in order of their increasing
energy ".
(ii) Uncertainty principles: -
· Given by: - Werner Heisenberg (1927).
· Principles: - It is impossible to measure the position &
momentum of a sub-atomic particle in an atom accurately. If p be the error
in measuring momentum & x be the error in measuring position of a
subatomic particles of an atom then according to Hisenberg's uncertainty
principles,
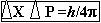
The uncertainty in measuring position & momentum of subatomic particles
occurs because light or electron beam has a finite & constant velocity
& its velocity can not be made equal to infinity.
Q 12 The radiator of a vehicle holds 10 L of water? If the vehicles
is to be used in Srinager where the lowest temperature is -10.0ºC
(263.15k), calculate the amount of ethylene glycol (molar mass = 62.1g
mol-1 ) which should be added to the water? (2 marks)
Ans. Here kf for water = 1.86 K kg mol-1 .
Molality (m) of a solution which freezes at 263.15 K .
m= T / kf = 10.0k
1.86 K Kg mol-1 = 5.4 mol Kg-1k kg mol-1
Since 5.4 moles of ethylene glycol is needed for 1 Kg of water
Therefore, 54 moles will be needed for 10Kg (i.e. mass of 10L)
=54.0X 62.1gm =335 X 101 of ethylene glycol.
Q 13.Define: -
(i) Osmosis
(ii) Osmotic pressure (2 marks)
Ans.
(i) If a solution is separated from the solvent by a semipermeable membrane,
which blocks the solute molecule but allows the passage of solvent molecules
from the solvent side to the solution side than from solution side to
the solvent side. This phenomenon which results in a net flow of solvent
to the solution is called Osmosis.
(ii) The passage of solvent through the membrane can be stopped if adequate
pressure is applied on the solution side. This is known as Osmotic pressure.
Q 14. Write IUPAC names of the following compounds: -
(i) CH3-CH2-O-CH2-CH-CH3
(ii) CH3-CH2-CH2-CO-CH3(2
marks)
Ans.
(i) 2,ethyl,propyl ether or Ethoxypropane
(ii) 1,Methyl, 2 propyl ketone or Pentanone.
Q 15.Starting from carbon to hydrogen how would you synthesis the
following compounds?
(i) Benzene
(ii) Formic acid.(2 marks)
Ans. ..........2500ºC ..................polymerisation

|