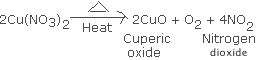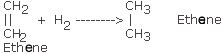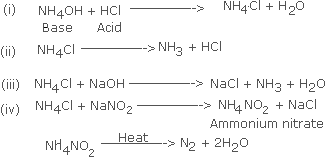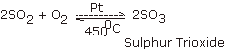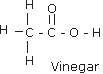ICSE Set Qa1 Year Icse Chemistry99 Qa1.php Chemistry Exam Paper for students online
|
Q2.(a) Choose the
Correct word or phrase from the bracket to complete the following
sentences : Q2.(b) Write the
correctly balanced equation for each of the reactions mentioned in
statements (i) - (iv) above.
Q2.(c) Describe what
you see when concentrated nitric acid is added to copper.
|
||||||||
| Q.3(a)(i)
What is the colour of the flame seen when sulphur burns in air ? (ii) Name the product formed when sulphur is burnt in air or oxygen.` (iii) When burning sulphur reacts with water, a compound is formed. Name the compound. (iv) Write the balanced equation for the reaction between sulphur dioxide and moist chlorine. (v) In the reaction mentioned in question (iv) above, which substance is the oxidizing agent ? Ans. 3 (a)(i) Sulphur in air at 250 to 3000C to give pale blue flame. (ii) Sulphur Dioxide (SO2) and traces of SO3 therefore S + O2 -- SO2 2S + 3O2 -- 2SO3 (7 to 8%) (iii) Sulphurous Acid SO2 + H2O -- H2SO3
(But Dry Chlorine gives SO2 + Cl2
-- SO2Cl2) Q.3(b)(i) What is the
purpose of the Contact Process ?
Q.3(c)(i) When hydrogen
sulphide reacts with oxidizing agents, what substance is always a product
of the reaction ? |
||||||||
Q.4(a) With reference to the reduction of copper oxide, iron (II) oxide, lead (II) and magnesium oxide by hydrogen, place the oxides in order of increasing ease of reduction. That is, put first the oxide that is most difficult to reduce ; and last, the oxide that is most easily reduced. Ans 4(a) MgO, FeO, PbO, CuO. Q.4(b) Write balanced
equations for the following reactions : Q.4(c)(i) What is the
type of bonding expected in a metallic chloride ? Q.4(d)(i) Cast iron
contains about 4% carbon. By what chemical process is the amount of carbon
decreased to make steel ? |
|
Q5.(a) Concentrated
nitric acid oxidises phosphorus to phosphoric acid according to the
following equation : (ii) For 98 gms/ H3PO4
º 63 x 5 gm/HNO3 is consumed (iii) For 98 gms H3PO4
º 18 gms of steam Q.5(b) Ammonia may be
oxidized to nitrogen monoxide in the presence of a catalyst according to
the following equation : |
||
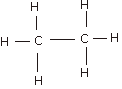
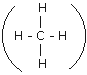
Q.6(a) For each of the compounds (i) Ethane, (ii) Vinegar and (iii) Marsh gas, draw the relevant structural formula. Ans.6 (a) (i)Ethane
(ii) Vinegar
or
(iii) Marsh Gas
Q.6(b)(i) What word is
used to describe these three compounds taken together ?
(iii) What type of reaction is common to
both these compounds ? |
||
Q7(a) Define the
following terms :
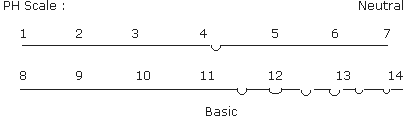
If pH value of a solution is lesser than 7,
it is Acidic in Nature (iii) Neutralisation is the process
in which H+ combine with OH- to form water or Acid react with a
base to form salt and water . Q.7 (b)(i) Outline the
steps that would be necessary to convert insoluble lead (II) oxide into
insoluble lead chloride. Ans7 (b) (iii) Fe + H2SO4
-------Fe 2SO4 + H2 (Ferrous Sulphate ,
Hydrogen) |